"Didn't go quite as described:" Women across the country, here in WI experience issues with Essure
MILWAUKEE -- It seemed to be the perfect solution. Little pain. No surgery. A quick recovery. But for some women -- using Essure permanent contraceptive has been a nightmare, and it's led to the very thing they were trying to avoid: surgery.

Kristy Conway
Kristy Conway's doctor told her about Essure, as an alternative to tubal ligation or getting her tubes tied. What she heard was appealing.
"It's non-surgical. They do it right in the office," Conway said.
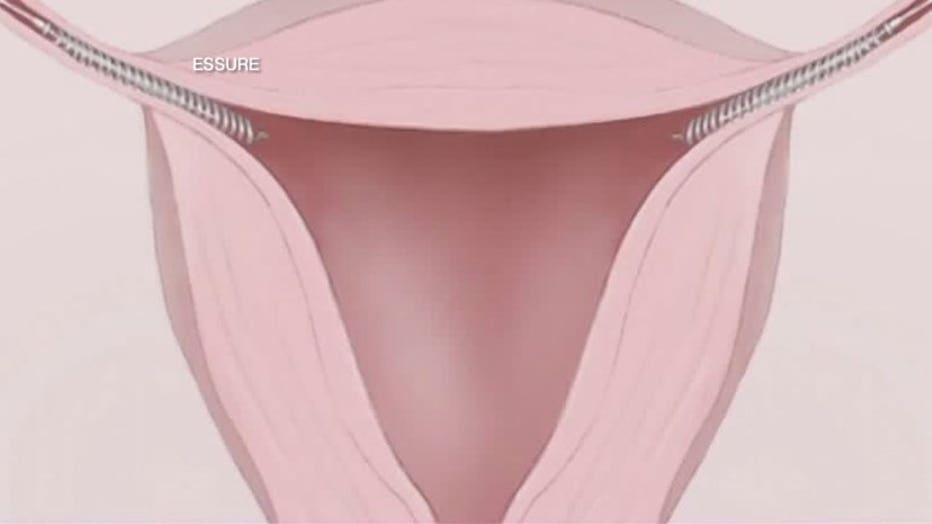
According to the manufacture of Essure, here's how it works. A doctor inserts two flexible metal coils into the fallopian tubes -- taking only minutes.
But Conway says that's not what happened in her case.
She says the procedure took almost two hours and there were complications in implanting the coils. The side effects Conway experienced are mirrored by thousands of women's descriptions on the "Essure Problems" Facebook page -- and subgroup, "Essure Victims of Wisconsin."
Essure
Side effects mentioned include pelvic and back pain, horrible headaches, severe anxiety, high blood pressure, fatigue, exhaustion... and in some cases, fetal death.

Essure
Dr. Edio Zampaglione, spokesman for Bayer, the parent company of Essure stated: "It is greater than 99 percent effective as long as the patient follows the process she has to go through."
As part of the procedure, patients must return to the doctor three months after insertion for a confirmation test to verify that the inserts are in the correct location and the body has formed a natural barrier around the inserts to prevent sperm from reaching the eggs.

Erin Kearns
Erin Kearns never made it that far.
"It didn't go quite as he described it," Kearns said.
After the procedure, the cramping didn't stop. Mild spotting turned into very heavy bleeding -- for six weeks. Two weeks after that, Kearns' doctor performed a hysterectomy, at the age of 28.
Referring to the "Essure Victims of Wisconsin" Facebook page Kearns said: "I wish I would have known more about it before I had it done. It is good to see I am not alone in what I had done."
In September 2015, the FDA held a hearing to review the Essure implant -- after getting reports of more than 7,000 adverse reactions, including pregnancy.
Essure
More than 750,000 of the devices have been sold worldwide.
In February, the FDA responded -- intending to require a boxed warning label and patient decision checklist. It also ordered parent company, Bayer, to conduct a post-market study for more data about Essure's benefits and risks.
But as Dr. Zampaglione emphasized, the FDA did not pull the product off the market.
"The FDA made it very clear that the product is staying on the market; that they believe this is an important option for women to have," Zampaglione said.
Essure
When the FDA approved Essure in 2001, it was given pre-market approval. That status shielded it from litigation.
Last month, Congressman Michael Fitzpatrick introduced a bill that would eliminate the litigation roadblock.
Meanwhile, women experiencing adverse affects from Essure are relieved to get hysterectomies to get the coils out.
"You know, I never had any issues after that," Kerns said.
If Fitzpatrick's bill becomes law, it would be retroactive to 1976, but we're told statute of limitations on the state level could still apply.
CLICK HERE to learn more about Essure.
CLICK HERE to visit the Essure Problems Facebook page.
CLICK HERE to visit the Essure Victims of Wisconsin sub-group on Facebook.

