Confused about COVID-19 vaccine and pregnancy? Doctors push for change

Confused about COVID-19 vaccine and pregnancy? Doctors push for change
Efforts to avoid experimenting on pregnant women in clinical trials are unintentionally making them part of an experiment in everyday life.
MILWAUKEE - Efforts to avoid experimenting on pregnant women in clinical trials are unintentionally making them part of an experiment in everyday life.
This conundrum puts pregnant health care workers first in line to make big decisions with little data about the COVID-19 vaccines, all while they are on the front lines of fighting a virus that is dangerous for expectant moms.
"There’s lots of [COVID-19-positive] pregnant women that did just fine in the hospital...but unfortunately, we had some pretty sad pregnant cases come through shortly after I got pregnant that really scared me," Kristen Wineland, an ICU nurse in Madison said in an interview with FOX6 when she was 39 weeks pregnant.
"One ended up needing an emergency C-section very early in her pregnancy and definitely wasn’t able to see her baby for a long time afterwards just because she was so sick and the baby spent a lot of time in the NICU," Wineland continued.

Wineland says her coworkers did their best to help limit her exposure to COVID-19 patients, but she couldn’t completely avoid it. With research showing pregnant women are at a higher risk for becoming seriously ill from COVID-19, but also revealing little information about how the COVID-19 vaccines affect pregnancy, Wineland found herself weighing a difficult decision.
"I thought about it a ton," Wineland said.
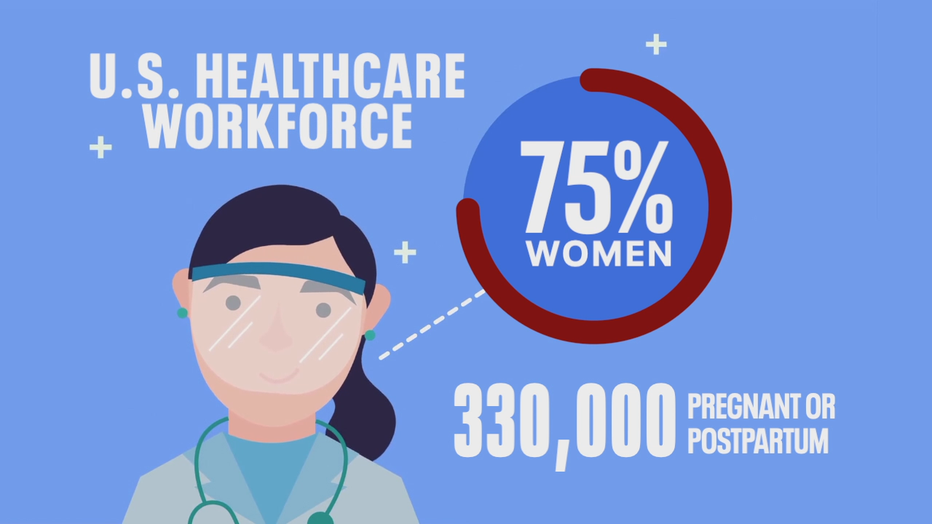
Wineland is not alone. The CDC says 75 percent of the U.S. health care workforce is female, with an estimated 330,000 expected to be pregnant or recently postpartum when they’re able to get the COVID-19 vaccine. But despite promising signs, doctors say they can’t give their pregnant patients the clear answers they'd like to provide about whether they should get this particular vaccine, even though they wish they could.
Researchers who study pregnancy say it doesn’t have to be this way; while they point to the conversation surrounding the COVID-19 vaccine as a step in the right direction, they are pushing for change in how medical research treats pregnant and breastfeeding women.
"The lag in having the right kind of evidence base is really astounding and quite frankly a tragic injustice," said Dr. Carleigh Krubiner. Dr. Krubiner is a policy fellow at the Center for Global Development and adjunct faculty at Johns Hopkins Berman Institute for Bioethics; she researches evidence gaps in pregnancy.
The history of excluding pregnant women
"Somebody had said to us, ‘Well, nobody wants to be the next Thalidomide dude,’" Krubiner said, describing the reluctance to include pregnant women in clinical trials.
Thalidomide is a sedative drug women used in the 1950s for morning sickness. It had not been tested on pregnant women; drug testing standards were different at the time, and Thalidomide had not gone through the more rigorous process in place today.

By the 1960s, Thalidomide had been linked to serious and life-threatening birth defects, prompting changes in drug testing standards.
Dr. Krubiner says Thalidomide is an extreme and rare example, and believes it would be erroneous to compare it to the development of vaccines, which are different biologic products than drugs. However, she says she’s heard others cite the "Thalidomide Tragedy" as a concern when it comes to including pregnant women in clinical trials, while her takeaway is the opposite.

Dr. Carleigh Krubiner
"What should have come from this is, ‘Oh, we had better be researching things in ways that make us more prepared to give informed decisions,’" Krubiner said, pointing to research that more extensive Thalidomide testing could have averted some of the most harmful effects and redacted the number of babies affected.
However, pregnant women routinely found themselves excluded from clinical trials. As a result, when other adults get the green light to take a new medication or vaccine, expectant moms often must wait years to get important information about safety.

Dr. Richard Beigi
"It has almost unfairly disadvantaged these women and I just don’t think it’s right," Dr. Richard Beigi, president of UPMC Magee-Womens Hospital in Pittsburgh, said.
Pregnancy weakens the immune system, making expectant moms more susceptible to negative outcomes from disease and epidemics. It's one reason the flu has disproportionately killed pregnant women. Yet even though the U.S. approved the flu vaccine in 1945, it took more than 50 years to get enough research to convince the CDC to recommend and prioritize flu shots for pregnant women in 1997.
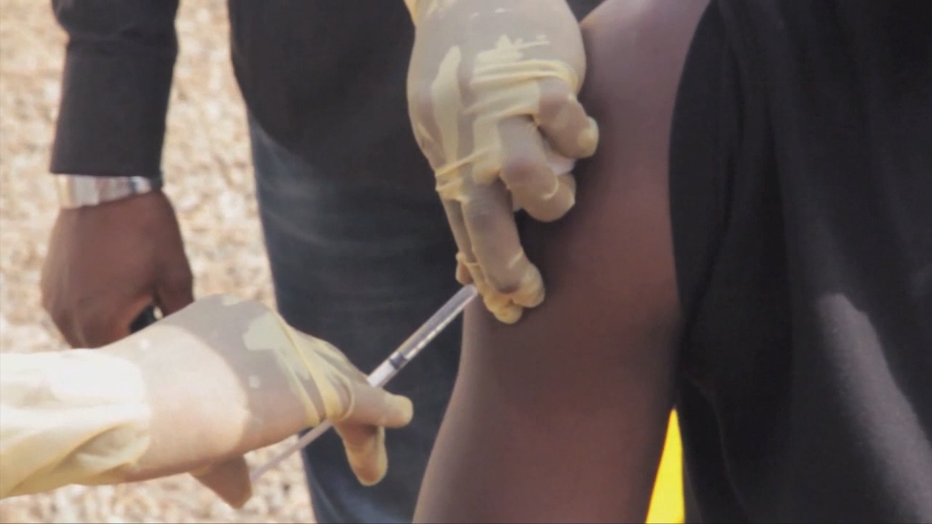
Ebola treatment and vaccine trials excluded expectant moms; researchers now say they were "protected to death," while those around them were able to receive efficacious vaccines.
Dr. Krubiner says limited data led to early theoretical concerns about vaccines that use a weakened form of the actual virus, such as rubella.
"In the early stages, it was actually advised that women may want to terminate their pregnancies if they had these exposures," Krubiner said. "And lots of women, either out of that recommendation or out of a fear of what this could mean unnecessarily terminated pregnancies when it turns out it was just fine."
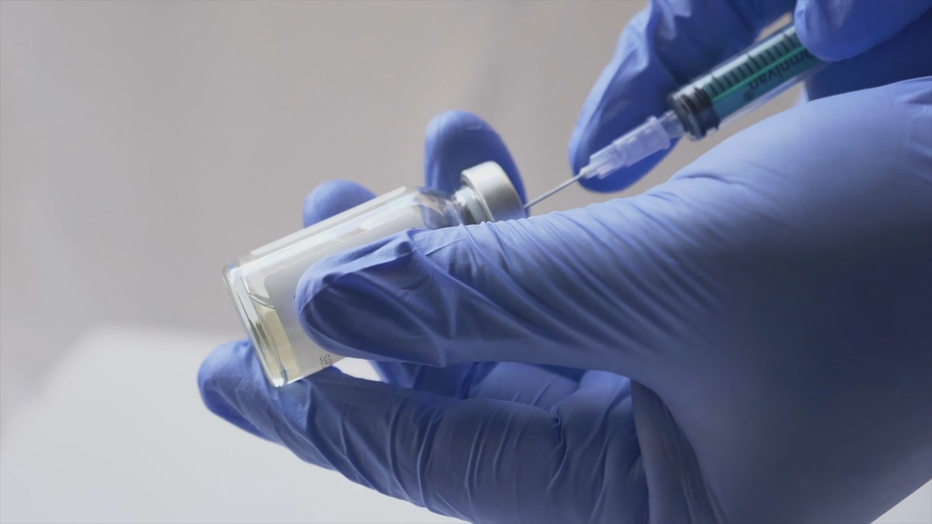
And a 2011 study found the average time for a drug to move out of an "uncategorized" risk in pregnancy was 27 years.
"Excluding pregnant women from research is doing a lot more harm than including them in responsible ways," Krubiner said. " It’s really appalling when you realize how pervasive this is and how deeply problematic this is."
Including pregnant women in ethical ways
Dr. Beigi and Dr. Krubiner are part of a Pregnancy Research Ethics for Vaccines, Epidemics, and New Technologies (PREVENT) working group that developed guidelines in 2018 for the ethical inclusion of pregnant women in vaccine trials.
The guidelines do not advocate for every pregnant woman to be a part of every clinical trial for every vaccine or medication; instead, the goal is to move away from automatically excluding pregnant women and to ensure they don’t get left behind as new products are developed.

Beigi and Krubiner say this would shift research away from the uncontrolled setting of the marketplace, in which pregnant women with more variables are making decisions with limited data, to the controlled setting of a clinical trial.
"We’d all be much better off if we had more robust databases in which to make more educated advice, and then women would feel more confident taking that advice," Beigi, who is also an obstetrician, said. "People will say, ‘We don’t have data so we shouldn’t study pregnant women,’ but how do you get the data if we don’t do thoughtful study?"

"Don’t start from a place of saying, ‘We can’t do this,’" Krubiner said. "Start from a place of, ‘Well maybe we can, but let’s see if there’s a reason we shouldn’t.’"
"People always say, ‘How are going to recruit pregnant women to the study?’" Krubiner continued. "Well, we’ve done it."
The COVID-19 vaccines
Despite the PREVENT guidance, the 2020 COVID-19 vaccine clinical trials excluded pregnant and breastfeeding women. While a handful of women in the trials received the vaccine before they knew they were pregnant and have reported no problems, doctors say the numbers are so few that they cannot form significant conclusions.
Trials with pregnant women are scheduled to begin later this month, and researchers say the available pregnant animal data looks promising.
"There’s no inherent reason to believe these vaccines will be harmful," Beigi said. "In fact, there’s every reason in the world to believe that they will be very helpful for pregnant women."

For now, pregnant women are currently able to have conversations with their doctors about the risks and benefits of the vaccines, but are unable to get a clear "yes" or "no" when they ask if they should receive it.
"It’s unfortunate that we don’t have the evidence base right now to say, ‘Yes, go get this,’" Krubiner said, noting that vaccines have a long history of safety in pregnancy.
"We would like to not be here," Begi said. "We would like to have a more robust body of information. But it kind of is what it is at this point."
Pregnant women who are not first in line to get the vaccine have time to watch more research develop. Pregnant health care workers, however, have the opportunity to get vaccinated now.
"I really thought, ‘Oh they’re probably not going to allow me to get this vaccine,’" Dr. Haley Spagnola, a pregnant pediatric hospitalist in Pennsylvania, said.
How to weigh the risks and benefits
Spagnola says she was pleasantly surprised when she saw guidance from the American College of Obstetricians and Gynecologists (ACOG). The guidance says pregnant women who decline the COVID-19 vaccine should be supported, but the vaccine should also not be withheld from pregnant patients.
"It is very challenging when you don’t have the data yet," Dr. Mark Turrentine, who helped develop the ACOG guidelines, said. "But then you have to base it on your past experiences with using vaccines in pregnancies."

Dr. Mark Turrentine
Turrentine says the goal of the ACOG guidance is to give pregnant women a choice with the COVID-19 vaccine, but he acknowledges it is a difficult choice to make in the absence of clear pregnancy research.
"We did not want to tell people, ‘You have to go get this’ because there’s too many unknowns at this stage," Turrentine said. "But we did want to provide the pluses and minuses."
Turrentine points out that COVID-19 dangerous, more so for pregnant women. He says pregnant patients who are 35 or older, overweight, have other medical problems, smoke, are in a racial or ethnic group at increased risk for the virus, in a community with a high rate of infections, in contact with people outside their households who do not wear masks, or health care workers "may be at a high risk of getting COVID, and it probably makes sense to get the vaccine."
Lost time, lost confidence
"They have to really sit down and assess their individual risk, and then weigh it against [a vaccine] we think is very safe, but we don’t have hard information to always show them," Turrentine said.
Researchers like Dr. Carleigh Krubiner and Dr. Richard Beigi praise the ACOG guidelines, believing that giving women a choice to get the COVID-19 vaccine instead of telling them to avoid it altogether represents a positive step forward.
However, they are concerned with delays in researching the effects on pregnant women will make it more difficult to inspire long-term confidence in the vaccine.
"If we already had, let's say, data on hundreds of women with both of these vaccines, this conversation would be fundamentally different," Beigi said. "And the net result of that most likely would be thousands to millions of more women would feel comfortable getting the vaccine, and then you have that much more disease prevention for the mother and the baby."

The flu shot is an often-cited cautionary tale. Although decades of studies confirmed the flu shot’s effectiveness and safety in pregnancy, the exclusion of pregnant women in earlier research led to delays in government organizations and advisory bodies fully endorsing and prioritizing the vaccine for pregnant women.
Now, the clear answers expectant moms say they wish they had about the COVID-19 vaccines are available about the flu shot; doctors enthusiastically encourage pregnant patients to get it. But CDC research says approximately half of pregnant women do not get the flu vaccine, with the most commonly-reported reasons being concerns about effectiveness and safety.
In other words, the longer doctors go without pregnancy research, the harder it is for pregnant patients to build trust in treatments and vaccines.
It’s why the pressure is now on pregnant health care workers.
The decision
Doctors say pregnant health care workers who initially decline the vaccine not are not foregoing their chance to get it; they’ll still be prioritized if they change their minds.
Lauren Huettner, a pregnant ER nurse in southeast Wisconsin, says she plans to wait until after she gives birth in the spring and is finished with pumping and breastfeeding.
"I have a history of a more difficult pregnancy, so I just don’t know how this would affect me now," Huettner said. "My coworkers are awesome because they’re trying their best to keep those of use who are pregnant out of COVID rooms. So I do feel safe at work."
Others, like ICU nurse Kristen Wineland, could only take small steps to limit their exposure to the virus.
"I thought about it a ton," Wineland said. "Being in the ICU and the procedures that we do, that aerosolizes the virus a lot for us in the room. My exposure is a lot higher than even just some other nurses."
Wineland said she had a "fantastic discussion" with her doctor, and decided she would get the COVID-19 vaccine. But she did not have a chance to get it before she went into quarantine ahead of the birth of her second child.
One week after she gave birth, Wineland received her first dose of the vaccine.
"The people who developed this stuff are brilliant and they’ve done a lot of work to get us to this point," Wineland said. "And I trust them."
"It was definitely a process for me," pediatric hospitalist Dr. Haley Spagnola, who works in Pennsylvania, said. "At the end of the day, if I have a sick asthmatic who is positive for COVID or a mom who is having a baby that’s positive, I still will take care of them and give them the same care I will give anyone else because they deserve that."
"Sometimes not doing something is also a risk," Spagnola added.

Spagnola got the vaccine at 24 weeks pregnant. She posted a picture on social media because she says it’s important to her that other pregnant women don’t feel alone while they’re trying to make this decision.
"As a health care worker who looks at this type of information in these types of settings all the time, I felt that way too," Spagnola said. "And you know that you’re not just making a decision for yourself, you’re also making a decision for your baby and the rest of your family...when I started to see pregnant physicians and other health care workers getting the vaccine, it made me feel like, ‘OK, I’m not this crazy person for saying, ‘I think i want to do this and I think this is the best choice for me and my baby."
Over the next several months, in addition to the planned clinical trials studying the COVID-19 vaccines’ effects on pregnancy, researchers will track results in pregnant health care workers who choose to receive the vaccines.
In other words, excluding smaller groups of pregnant women from research in the controlled environment of a clinical trial has led to research on larger groups of pregnant women in the less controlled setting of publicly-available vaccinations.
"Unfortunately, a lot of people see pregnant women and breastfeeding women as these nebulous-like aliens," Spagnola added. "‘Oh no, they’re really scary; just don’t do it. Don’t take that medication, don’t get the vaccine, that’s what the safest option is.’ And often that’s not true."
Read the ACOG guidance about the COVID-19 vaccine, pregnancy, and lactation.
Featured
Pharmacy license suspended for Wisconsin man accused of ruining vaccine
The Wisconsin Pharmacy Examining Board voted unanimously to suspend the license of a former Aurora Grafton pharmacist accused of intentionally sabotaging the COVID-19 vaccine.
Featured
DHS: 2,134 new positive cases of COVID-19 in WI; 37 new deaths
As for the coronavirus vaccine, state health officials announced Wednesday, Jan. 13, that 176,165 doses have been administered.
Featured
Contagious COVID-19 variant detected in Eau Claire County resident
The Wisconsin Department of Health Services and laboratory partners identified a variant strain of SARS-CoV-2, the virus that causes COVID-19, in Wisconsin




